Therapeutic Enhancement of Polymer-Drug Conjugates via Induction of Crosslinking-Mediated Endocytosis
Polymer-drug conjugates have great potential as anticancer agents. However, the efficacy of anticancer polymer-drug conjugates relies not only on their increased accumulation in the tumor tissue, but also their ability to subsequently deliver drugs to intracellular targets. Therefore, there are two distinct, yet complementary means to optimize efficacy of polymer-drug conjugates: improve pharmacokinetics to increase accumulation in the tumor tissue, and/or improve the endocytic efficiency – the fraction of conjugates in the tissue that is actually internalized by cells and allowed to exert their therapeutic effect.
Previously we have developed long-circulating “second-generation” polymer-drug conjugates that marry enhanced tumor tissue delivery with excellent biocompatibility in vivo [1]. This project aims to augment that system with active targeting ligands to enhance intracellular delivery with the goal of driving a concomitant enhancement of therapeutic efficacy.
While active targeting is a common strategy to enhance the uptake of drug carriers, there are many therapeutically-relevant receptor targets that do not induce internalization upon ligand binding. However, many of these receptors have been shown to be susceptible to a different endocytosis mechanism – crosslinking mediated endocytosis. In this approach, hyper-crosslinking of the cell surface receptors overrides their native behavior, leading to rapid internalization and subsequent lysosomal trafficking of both the carrier and the receptor. Interestingly, this mechanism has been reported for a number of different receptors across cell lines. As such, it appears to be a general endocytosis pathway that could be applicable to a number of clinically-relevant receptor targets.
We aimed to co-opt this strategy, developing multivalent N-(2-hydroxypropyl) methacrylamide (HPMA) copolymer-based nanomedicines rationally-designed to exploit crosslinking-mediated endocytosis for improved endocytic efficiency. We have successfully validated this strategy using multivalent conjugates presenting ligands against “internalizing-resistant” cancer-specific receptors such as HER2, enhancing intracellular delivery and cytotoxic activity towards cancer cells overexpressing the target receptor [2]. In one instance, lead candidates achieved high intracellular delivery even at picomolar treatment concentrations; untargeted HPMA copolymers required 1000-fold higher treatment concentrations to achieve similar levels of intracellular accumulation.
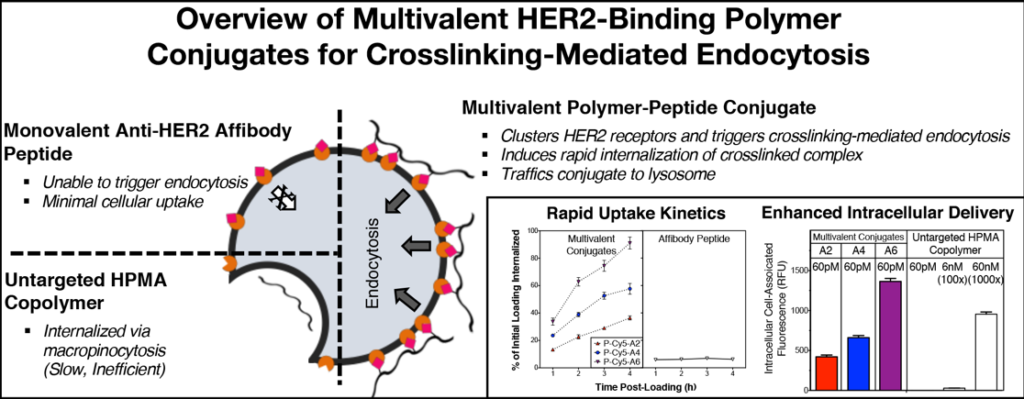
Additionally, as this strategy traffics both carrier and receptor to the lysosome, we have also applied it to deplete pathological cell surface receptors. One such application was utilizing a multivalent polymer-peptide agonist against PD-L1 as a novel checkpoint blockade immunotherapy [3]. By durably depleting the receptor in the tumor microenvironment (rather than the transient blocking afforded by traditional checkpoint antibodies), we were able to produce enhanced anti-tumor immunity in vivo.
